| Laboratory: | Argonne National Laboratory |
| Capability Expert(s): | Debbie Myers, Nancy Kariuki, and A. Jeremy Kropf |
| Capability Details: | |
| Title: | In Situ and operando atomic and nanostructure characterization |
| Class: | Characterization |
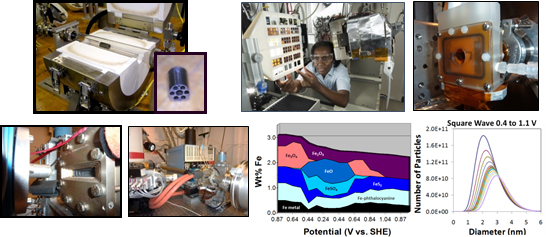
| Description: | This capability utilizes a combination of synchrotron X-ray spectroscopy, microscopy, and scattering and custom-built cells to determine the atomic structure, oxidation state, and nano- and micro-structure of catalysts, catalyst-ionomer inks, and electrodes in various environments. Briefly, the X-ray techniques and the information they provide are:
These techniques can be applied to catalyst materials, catalyst precursors, catalyst-ionomer inks, and electrodes, and, for all techniques but tomography, electrodes in aqueous or membrane electrode assembly (MEA) environments. They can also be performed under controlled temperature, atmosphere (e.g., during pyrolysis), humidity, and potential conditions. The apparatuses available are two aqueous electrolyte cells, single-cell MEA hardware (and test stand), and a multi-port high-temperature sample holder and associated tube furnace and gas manifold. Examples of the use of these techniques for fuel cell catalyst and electrode characterization are determination of:
|
| Capability Bounds: | The Advanced Photon Source (APS) is a hard X-ray source and thus probes atomic structure via excitation of atoms with atomic numbers higher than 20 (e.g., at the transition metal X-ray absorption edges). X-ray absorption is a bulk technique and cannot distinguish between species participating in the electrochemical reaction or “spectator” species. Special techniques, such as delta-µ, may be able to resolve the species formed on the reactive surfaces of catalysts if these species represent a significant fraction of those absorbing at any given X-ray energy. For operando X-ray absorption studies of a fuel cell electrode, the electrode on the opposing side of the cell should not contain the material of interest (i.e., studies of a Pt cathode typically utilize an alternative anode material, such as Pd). Nano-X-ray computed tomography and microscopy require small sample sizes (~10 µm in diameter). |
| Unique Aspects: | The cells and associated apparatuses have been developed and are unique to Argonne. The capability to use these cells in combination with all the X-ray techniques is also unique to Argonne. The high brilliance and the penetration depth of the hard X-rays of the APS allow probing “buried” interfaces such as fuel cell electrodes encased in carbon or surrounded by an aqueous electrolyte. |
| Availability: | The cells were developed and are the property of the Chemical Sciences and Engineering Division at Argonne and are fully available for DOE-FCTO consortia use. Argonne is home to the Advanced Photon Source (APS) synchrotron light source which is a DOE Office of Science user facility. Time on the appropriate APS beam line is available either by directly applying to the APS (https://www1.aps.anl.gov/users-information/about-proposals/apply-for-time) or coordinating the time through Deborah Myers. The cells and associated instrumentation (e.g., potentiostats, test stand, etc.) are available by contacting Deborah Myers. |
| References: |
J.A. Gilbert, A. J. Kropf, N.N. Kariuki, S. DeCrane, X. Wang, S. Rasouli, K. Yu, P.J. Ferreira, D. Morgan, and D.J. Myers, “In-Operando Anomalous Small-Angle X-Ray Scattering Investigation of Pt3Co Catalyst Degradation in Aqueous and Fuel Cell Environments,” Journal of The Electrochemical Society, 162 (14) (2015) F1487-F1497. M. Ferrandon, X. Wang, A.J. Kropf, D.J. Myers, G. Wu, C.M. Johnston, and P. Zelenay, “Stability of iron species in heat-treated polyaniline–iron–carbon polymer electrolyte fuel cell cathode catalysts,” Electrochimica Acta, 110 (2013) 282-291. M. Ferrandon, A.J. Kropf, D.J. Myers, K. Artyushkova, U. Kramm, P. Bogdanoff, G. Wu, C.M. Johnston, and P. Zelenay, “Multitechnique Characterization of a Polyaniline-Iron-Carbon Oxygen Reduction Catalyst,” Journal of Physical Chemistry C, 116(30) (2012) 16001-16013. D. Myers, X. Wang, D.J. Liu, N. N. Kariuki, and R. Ahluwalia, “Rationally Designed Catalyst Layers for PEMFC Performance Optimization,” FY 2015 Progress Report for the Hydrogen and Fuel Cell Technologies Office, U.S. Department of Energy. |
| Benefit: | The activity of catalysts electrochemical reactions depends on their atomic-level properties. These properties include oxidation state, particle size, local atomic environment of the reactive site, electronic structure, interatomic bond lengths, and atomic order. Characterization of these properties, especially while the catalyst is in the reactive environment, allows the correlation of these properties with activity, identification of the active catalyst site, an understanding of degradation mechanisms, and for improvement of the catalytic activity and ultimately fuel cell performance and durability. |