| Laboratory: | Los Alamos National Laboratory (LANL) |
| Capability Expert: | Edward F. Holby, [email protected], (505) 665-0034 |
| Capability Details: | |
| Title: | Modeling of PGM-free Electrocatalyst Materials |
| Class: | Computational Tools |
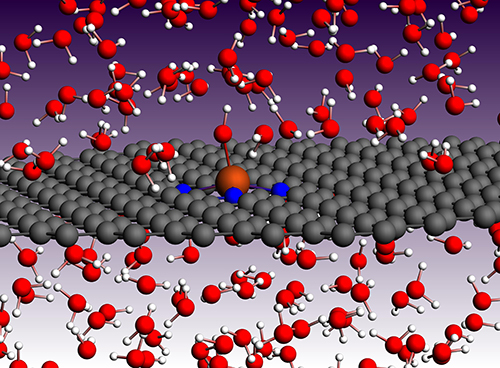
| Description: | Modeling of structure:function relationships for electrocatalyst materials provides valuable input for guiding the synthesis of catalysts with improved activity, selectivity, and durability, as well as aiding in the interpretation of experimental data. Such efforts are of particular importance for Platinum Group Metal free (PGM-free) electrocatalysts where synthesis approaches produce a heterogeneous material structure. This heterogeneity can make it difficult to attribute improved properties to specific structural components, which is a major barrier to targeted material optimization. In particular, the atomic scale structure of the active site(s) can greatly affect reaction pathway (and thus activity/selectivity) and corrosion susceptibility (via both corrosion and poisoning). Quantum chemical modeling tools are well suited for studying these structure:function relationships providing information regarding relative stability, reaction pathways, effects of ligands and poisons (useful for active site quantification via probe molecules), and the thermokinetics of structural degradation. These tools include density functional theory, ab initio molecular dymamics, density functional tight binding, and reactive force field based molecular dynamics. These tools enable high-throughput based approaches to materials optimization. Calculated atomic scale properties are used to inform higher time/length scale models such at Lattice-Boltzmann and kinetic reaction rate models required for capturing mass transport effects in electrodes. Additionally, detailed atomic information is beneficial for interpreting a variety of spectroscopic experimental approaches. These modeling approaches have been applied to nuclear resonance vibrational spectroscopy (NRVS), Mössbauer spectroscopy, X-ray Absorption Fine Structure (XAFS) spectroscopy, in-situ electrochemical δμ technique, and X-ray Photoelectron Spectroscopy (XPS). Prediction of spectroscopic fingerprints (with and without probe molecules) via modeling can fill important gaps in data sets used for fitting spectroscopic data in heterogeneous materials. Combined, these approaches add great value to understanding what makes ideal active site structures, what structures exist in synthesized materials, and how synthesis can be improved to achieve more optimal PGM-free catalyst materials. |
| Capability Bounds: | The capabilities listed above span a wide range of time/length scales relevant for catalysts. Due to the multi-scale nature of the combined approaches, the nanoscale and mesoscale are directly modeled and further interpolation to macroscale properties (measured reaction and degradation rates) are possible. |
| Unique Aspects: | LANL’s world-class research in the field of electrocatalysis is based on decades of research expertise coupled with dedicated computational and experimental resources. LANL has a wide variety of in-house synthesis, characterization, and modeling capabilities, which are integrated via close collaborations. |
| Availability: | LANL’s Institutional Computing provides world-class, state-of-the-art, high performance computing facilities to LANL researchers and collaborators. |
| References: |
Chung, Cullen, Higgins, Sneed, Holby, More, and Zelenay, “Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst,” Science 357 (2017).Holby and Zelenay, “Linking structure to function: The search for active sites in non-platinum group metal oxygen reduction reaction catalysts,” Nano Energy 29 (2016). Martinez, Dumont, Holby, Artyushkova, Purdy, Singh, Mack, Atanassov, Cullen, More, Chhowalla, Zelenay, Dattelbaum, Mohite, and Gupta, “Critical role of intercalated water for electrocatalytically active nitrogen-doped graphitic systems,” Science Advances 2 (2016). Holby and Taylor, “Activity of N-coordinated multi-metal-atom active site structures for Pt-free oxygen reduction reaction catalysis: Role of *OH ligands,” Scientific Reports 5 (2015). Holby, Wu, Zelenay, and Taylor, “Structure of Fe-Nx-C Defects in Oxygen Reduction Reaction Catalysts from First-Principles Modeling,” The Journal of Physical Chemistry C 118 (2014). |
| Benefit: | LANL’s existing catalyst modeling capabilities will directly benefit ElectroCat via improved understanding of how material structure impacts electrocatalyst performance (activity, selectivity, and durability). This understanding will help guide targeted synthesis in order to accelerate development of optimized PGM-free electrocatalysts. |
| Robustness: | While progress has been made in the area of understanding how atomic scale structure impacts PGM-free electrocatalyst behavior, four areas of research will aid in improving the utility of these approaches. These include directly linking synthesis environment to synthesized structures, qualification of ab initio molecular dynamics durability descriptor (underway), coupling of mesoscale effects to active site structures to better understand mass transport and solvation effects on activity, and automated high-throughput methods for generating computationally obtained databases of active site signatures (including activity descriptors, durability descriptors, and spectroscopic signatures). |